
SAFETY
It doesn‘t make much sense to learn about refrigeration if you‘re going to get yourself killed or so injured that you can‘t work on it or anything else - or if you endanger others. When you work around the refrigeration system - or any part of the HVAC system - remember these principles:• You are dealing with electricity, heat, pressures, and possibly toxic gases. Don‘t start or stop equipment, touch tubing, adjust valves, or open lines unless you really know what you are doing.
• Almost all HVAC equipment operates automatically - it can start at any time. Always be aware of this.
• If you are working around any powered equipment,lock outandtag outthe switch to be absolutely certain that the power is off and cannot be turned on by anyone but you:
- Turn off the main disconnect switch to the equipment.
- Lock the switch with your padlock and tag it with your name and the date.
- Even though you have switched off the equipment, test the circuit to be sure the power is off. Sometimes strange things happen with the wiring of electrical circuits.
Never attempt to service any refrigeration system unless you are qualified and certified. The high side of the refrigeration cycle can be very hot, and you can be burned if you touch any uninsulated parts. Opening a system can be very dangerous because it can freeze your skin. In addition, some refrigerants are harmful to the atmosphere, so it is illegal to release them. You must not handle them unless you have passed a certification test.

TEMPERATURE
Fahrenheit and Celsius ScalesTemperatureis a measure of the degree of heat contained in a substance. Canada and almost every other country except the United States use the Celsius (°C) temperature scale. In the United States, Fahrenheit (°F) is still the official temperature scale, but more and more, you see both °F and °C given together. Eventually, the United States will convert to the metric system and use the Celsius (°C) scale.
Even though I have grown up with the Fahrenheit scale and know what the temperatures feel like, I have to admit that the Celsius scale makes a lot more sense. In the Celsius scale, water freezes at 0°C and boils at 100°C. There’s logic to that. In the Fahrenheit scale, water freezes at 32°F and boils at 212°F. There’s no logic to that. If you work with the Celsius scale, you will soon learn that you are comfortable at 20°C and feel too hot at 30°C. The Celsius and Fahrenheit scales are shown in Figure 1. The table below compares some common temperatures:
Usually you will work in either °C units or °F units and there is no need to convert. If you are in situations where you need to convert, the simplest way is to carry a conversion chart in your toolbox for the normal range of temperatures. You can also record these equations in your notebook:

Figure 1. Compare the Celsius scale with the Fahrenheit scale. (Click on the image for an enlarged view.)
°F = (1.8 x °C) + 32
• To change Fahrenheit to Celsius:
°C = 0.56 x (°F –32)
For example, what is the temperature in Fahrenheit if the room temperature is 21°C?
°F = (1.8 x °C) + 32
°F = (1.8 x 21°C) + 32
°F = 69.8
What is the temperature in Celsius if the room temperature is 78°F?
°C = 0.56 x (°F - 32)
°C = 0.56 x (78°F - 32)
°C = 25.8

Figure 2. The Kelvin scale and Rankine scale are used for scientific and engineering work. (Click on the image for an enlarged view.)
You may hear references to the Rankine and the Kelvin temperature scales, so we mention them here. About all you need to know about them is that they are used in scientific and engineering work.
Both of these scales haveabsolute zeroas their minimum point. Absolute zero is the theoretical temperature at which a substance would contain no heat and the molecular particles in the substance are not moving. In theory, that’s as low a temperature as can be reached. The Kelvin scale uses the same graduations as the Celsius scale. The Rankine scale uses the Fahrenheit graduations (Figure 2).
Ambient Temperature
You will hear the termambient airorambient temperature, such as, “the ambient temperature should not exceed 104°F (40°C).” In HVAC work it means the temperature of the air surrounding the equipment.
HUMIDITY
Humidity is the amount of moisture in the air. In air conditioning we are concerned with the relative humidity of the air.Relative humidity (rh)is the percent of moisture in the air as compared to 100 percent of the moisture that air at that temperature can hold:• An air measurement of 70°F, 50 percent rh means that the air contains 50 percent of the moisture that the air is capable of holding at 70°F.
The amount of moisture that air can hold varies with the temperature. The warmer the air is, the more moisture it can hold. For example, suppose that air at 60°F has an rh of 50 percent. If the temperature rises to 80°F, the rh will decrease to 27 percent. The relative humidity has gone down, but theamountof moisture in the air remains the same.
Relative humidity is as important as temperature in creating comfort conditions in air conditioning. Generally, people feel more comfortable in a range of 40 percent to 50 percent rh.
HEAT
Above absolute zero, every substance contains some heat. Things that feel cold still contain heat. It‘s just that the amount of heat they hold (as measured by temperature) is less than the temperature of our body. For example, ice contains some heat - but it contains less heat than the same amount of water.The unit of heat used in the United States is theBritish thermal unit (Btu). The unit of heat in the metric system is thejoule, or thekilojoule (kj), which is 1,000 joules:
• A Btu is the heat that will raise the temperature of one pound of water 1°F. It is roughly equal to the heat you would get by totally burning a wooden match.
• A kilojoule is the rough equivalent of one Btu. To be more exact, one Btu is 1.055 kj.
You may want to remember these equivalents:
• 1 Btu = about 1kj = Heat needed to raise 1 lb. of water 1°F = About the heat of burning one wooden match.
The capacity of refrigeration units is often rated intons. For example, a chiller for a medium-size commercial building might be rated at 200 tons. The termtons of refrigerationis also used to refer to the cooling load on a room or building. Oneton of refrigerationis the amount of latent heat absorbed when one ton of ice at 32°F melts to water at 32°F during 24 hours. (Latent heat is explained later in this article.) One ton of refrigeration equals 12,000 Btu per hour (Btuh).
Delta T, ∆T
The difference between two temperatures is called the∆T, pronounceddelta T. A technician might take the air temperature on the upstream and downstream sides of a cooling coil. The difference between the two temperatures is the ∆T.
HEAT FLOW
There is a saying, “Heat flows downhill.” This means that heat always flows from a warmer to a cooler solid, liquid, or gas. The greater the ∆T, the faster the heat flow. Heat flow is a basic principle of refrigeration - in fact, of all HVAC work. For example, in the refrigeration cycle, the evaporator (cooling coil) is colder than the air flowing around it, so heat flows from the warmer air into the evaporator. The air-cooled condenser in the cycle is hotter than the ambient air, so heat flows from the hot condenser to the cooler ambient air.HEAT TRANSFER
We just talked about heat flow.Heat transferis themethodby which heat flows. This is another basic principle of refrigeration. The evaporator transfers heat into the refrigerant; the refrigerant transfers this heat to the condenser; the condenser transfers the heat to a cooling medium (such as water or outside air).Heat is transferred by:
• Conduction
• Convection
• Radiation
Conduction
Conduction is the flow of heat through a substance by contact of particles. If you hold one end of an iron bar and heat the other end with a torch, soon the end you are holding will become too hot to hold. This is because heat has transferred from one end of the bar to the other by conduction. Heat from hot refrigerant transfers by conduction through the metal of a condenser coil to the outside of the coil.
Conductivity is the ability of a substance to transfer heat by conduction. We are concerned here with heat, but you should know that conductivity also means the ability of a substance to carry an electric current. A material with high conductivity transfers heat well. Copper is used in the parts of a refrigeration unit that transfer heat because it has high conductivity. Insulation has low conductivity because it resists the flow of heat.
Convection
Convection is basically the transfer of heat by movement of a gas or a liquid. The heat that travels through the walls of a condenser coil by conduction is carried into the air by convection. The heat from HVAC supply air that is delivered into a room through an air outlet moves throughout the room by convection, moving on air currents.
Radiation
Radiation is the transfer of heat by electromagnetic energy. The rays from the sun turn into heat when they strike an object that they cannot pass through. On a sunny day, any metal exposed to the sun will become hot to the touch because of heat transfer by radiation.
Heat Transfer in HVAC Work
An HVAC system uses all three methods of heat transfer in many ways. For example:
• On a sunny day, the sun’s rays heat the outside of a building by radiation.
• This heat flows through the walls of the building by conduction to the interior surface of the outside wall.
• The heat from the wall is transferred to the air in the building by convection.

SPECIFICS
When you see the word specific in terms likespecific heat,specific volume, andspecific gravity, you know that it is a means of comparing values to a standard.Specific Heat
The specific heat of a substance is the heat required to raise one pound of the substance 1°F. Thespecific heat of water is 1. This means that 1 Btu will raise the temperature of one pound of water 1°F.
The specific heat of liquid R-134a refrigerant is 0.34. This means that only 0.34 Btu is required to raise the temperature of one pound of R-134a refrigerant 1°F. The specific heat of standard air is 0.24 Btu per pound per degree Fahrenheit.
In the metric system, specific heat is stated as the amount of heat that must be added to one kilogram of the substance to raise the temperature 1°K (Kelvin). The specific heat of water is 4.187 kj/kg °K (kilajoules per kilogram, Kelvin).
Specific Volume
The specific volume of a gas is the volume in cubic feet of one pound of a gas at standard conditions (70°F at 14.7 psi). Compare the specific volumes of the following three gases:

Specific volume in the metric system is the volume in cubic millimeters of one kilogram of the gas at standard conditions.
Specific Gravity
Specific gravity is the ratio of the weight of a liquid or solid as compared to an equal volume of water. The specific gravity of water is 1. Any solid or liquid that has a specific gravity of less than 1 will float on water. Compare the specific gravities of the following three substances:
These figures show that gasoline is lighter than water so it will float on water. Muriatic acid is heavier than water so it will sink to the bottom of a container of water.
PRESSURE
To understand the refrigeration cycle, you must understand pressure and the terms used with pressure. In the HVAC industry, pressure is measured in three ways:• psi - pounds per square inch
• inches Hg - inches of mercury
• inches wg - inches water gauge
Pounds Per Square Inch - psi
You know the term psi (pounds per square inch) because you encounter it daily. It‘s the pressure you measure for the air in your car tires. The same unit is used to measure the pressure of the air around us. The air above us has weight. At sea level, the weight of the air exerts a pressure of 14.7 psi. This is calledatmospheric pressure.

Figure 3. Simplified mercury barometer. (Click on the image for an enlarged view.)
Inches of mercury (inches Hg) is used to measure small amounts of pressure, especially negative pressures. It indicates how high a pressure will push mercury up in a tube. Figure 3 shows the principle:
• An open vessel contains mercury.
• A glass tube is inserted into the mercury. The bottom of the tube is open and the top is closed.
• All of the air is evacuated from the tube to create a vacuum.
• The atmospheric pressure at sea level will push the mercury up into the tube to 29.92 inches. This is the same as the atmospheric pressure of 14.7 psi.
In refrigeration work, inches Hg (inches of mercury) is used to measurenegative pressures, calledvacuums. A vacuum is a negative pressure (pressure below zero) and is indicated by the word vacuum, such as, 4.5 inches Hg vacuum (or Hg vac). The principle of a negative pressure is shown in Figure 4:

Figure 4. Measuring negative pressure in inches Hg vacuum. (Click on the image for an enlarged view.)
• The glass tube is open at the top and connected to a tank by a hose.
• When a vacuum pump draws the air out of the tank, a negative pressure is created.
• This negative pressure draws the mercury up into the tube. In Figure 3, the vacuum in the tank has drawn the mercury up to the 4.5 inch mark, so the vacuum in the tank is read as 4.5 inches Hg vac.
Mercury is a hazardous material, so in daily practice, other gauges are used to determine negative pressures. However, measurements are still indicated in inches Hg. A compound gauge can be used to measure pressure greater than zero in psi as well as pressures less than zero in inches Hg vacuum.
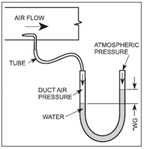
Figure 5. U-tube to measure pressure in inches wg. (Click on the image for an enlarged view.)
Inches water gage (inches wg) can be used to measure small pressures. This measurement is used to measure the pressures in air conditioning ducts. It uses the same principle as inches Hg. The method is shown in Figure 5:
• A tube in the air duct senses the pressure.
• This pressure is connected to a U-shaped glass tube that is filled with water.
• The pressure pushes the water higher on the open side.
• The difference in height of the two water columns is the pressure in inches wg.
Comparison of Pressure Units
The three pressures are compared below:

Pascal’s Law says that if you apply pressure to a fluid (liquid or gas) in a confined container, the fluid will apply the same pressure in all directions. This is why hydraulic systems work. Refrigeration systems also operate on this principle. The refrigerant liquid and vapor fill the available space.
You will not need to use Pascal‘s Law, but you should know what it refers to.
PRESSURES YOU NEED TO KNOW
Pressure is pressure - right?WRONG!You will be dealing with three different pressure measurements.• Atmospheric pressure
• Gauge pressure (psig)
• Absolute pressure (psia)
Atmospheric Pressure
Atmospheric pressure is the pressure placed on us by the atmosphere. Air has weight, and atsea level, the weight of the air above us results in a pressure on us of 14.7 psi (or 29.92 inches Hg).
Elevationaffects atmospheric pressure. As we go higher above sea level, there is less air above us and therefore less pressure. At 5,000 feet elevation, atmospheric pressure is 12.23 psi (compared to 14.7 psi at sea level).
Temperaturealso affects atmospheric pressure. As air heats up, it expands and therefore weighs less. (That is why hot air tends to rise.)
Standard airis dry air at sea level with a temperature of 70°F. It has a pressure of 14.7 psi. For air conditioning calculations, the value of standard air is used for altitudes up to 2,000 ft. and for temperatures between 40°F and 100°F.
Variations in pressure are important for refrigeration work. Refrigerant changes state (boils) at a lower temperature when it is at a higher elevation which has less atmospheric pressure.
Gauge Pressure
The pressure you see on gauges is the pressure above or below atmospheric pressure. Gauge pressure is the pressure in a system (apart from atmospheric pressure). It can be indicated by the termpsig(pounds per square inch gauge). In other words, 100 psig is 100 psi greater than atmospheric pressure.
The pressure reading for a gauge is generally given as psi, although it is understood that it means psig (gauge pressure).
Absolute Pressure
Absolute pressure is gauge pressure plus atmospheric pressure. It is indicated by the termpsia(pounds per square inch absolute). A gauge pressure of 100 psig is the same as 114.7 psia (100 psi + 14.7 psi atmospheric pressure).
PRESSURE AND TEMPERATURE
A change in pressure affects the temperature at which a liquid changes state (boils or condenses). The pressure-temperature relationship is an important principle for refrigeration.As pressure decreases, the change-of-state temperature also decreases. For example, at sea level, water boils at 212°F. At 5,000 feet, water boils at 202°F. This principle is applied in the refrigeration system:
• When the pressure is increased, refrigerant condenses or boils at a higher temperature.
• When the pressure is decreased, the refrigerant condenses or boils at a lower temperature.
The relationship between pressure and temperature allows the refrigeration system to make use of thelatent heatabsorbed or released by a change of state.
LATENT HEAT
There are threestatesof a substance:• Solid
• Liquid
• Gas
For example, the three states of water are:
• Solid - Ice
• Liquid - Water
• Gas - Steam or vapor
The termvaporcan mean the same asgas, or it can mean a gas with droplets of liquid suspended in it (also called wet vapor). The term vapor is used with refrigerants in the refrigeration cycle.

Figure 6. Latent heat for water. (Click on the image for an enlarged view.)
Latent heatis the heat released or absorbed by a substance when it changes state, with no change in temperature. For example:
• When steam at 212°F condenses to water, 970 Btu/lb is released.
• When water at 212°F boils to steam, 970 Btu/lb is absorbed.
If a pound of water is heated to 212°F, it will start to turn to steam. The temperature of the steam will be the same as the water - 212°F. When the entire pound of water has evaporated to steam, it will contain an additional 970 Btu. If you let the steam cool, it will condense back to water and it will have released 970 Btu/lb.
When ice changes to water or water changes to ice, a latent heat of 144 Btu/lb is absorbed or released by the same process:
• When water at 32°F changes to ice at 32°F, 144 Btu/lb is removed.
• When ice at 32° changes to water at 32°, 144 Btu/lb is added.

Figure 7. Types of latent heat. (Click on the image for an enlarged view.)
• Latent heat of vaporization
• Latent heat of condensation
Latent heat is the basis of refrigeration, because it means that relatively large amounts of heat can be transferred using relatively small changes in temperature.
Each substance has different latent heat values. For example, compare the latent heat of vaporization for water and Refrigerant R-134a:

HEAT OF COMPRESSION
When a gas is compressed, the energy used to compress it is turned into sensible heat and is absorbed by the gas. This is the heat of compression. In the refrigeration cycle, the compressor adds pressure to the low-pressure refrigerant vapor that comes from the evaporator. This adds the heat of compression to the refrigerant, and this heat is transferred from the refrigerant into the air by the condenser.ENTHALPY
For all practical purposes, enthalpy is thetotal heatcontent of a substance:• The enthalpy of a liquid = the sensible heat at that temperature.
• The enthalpy of a vapor = the sensible heat plus the latent heat that was required to form the vapor at that temperature.
Engineers use enthalpy in design work, but it is not used in the field.
Excerpted and reprinted fromRefrigeration for HVAC Techniciansby Leo A. Meyer, one of the books in the Indoor Environment Technician’s Library series published by LAMA Books.
Publication date:05/21/2007






