There is a large body of publications regarding uses of cast iron. But very few of them are concerned with the use of cast iron in refrigeration equipment, and even less with the behavior of such materials at low temperatures. This is mainly because there is the tendency to confuse the properties of cast iron pieces at normal and low temperatures. Some studies concerning the use of cast iron items at low temperature were made in the military field, but these studies were generally not accessible to the public, since they were considered secret information.
The purpose of this three-part series is to attempt to provide answers to several questions that may arise during the process of designing cast iron items for industrial refrigeration equipment:
• Is cast iron compatible with the refrigeration field?
• Does the piece present a full guarantee of operation?
• To what extent can cold shortness be estimated?
• Does the chemical composition fill the requirements of the operating conditions?
• How can we obtain gas-proof pieces?
• Is the construction of the piece functionally, technologically, and economically suitable?
Besides the custom conditions that must be satisfied by the cast iron item when used within refrigerating equipment (e.g. crankcase, arming-body, protecting-cap of heat exchanger, loose-flange, and others), there is an additional set of special conditions which also have to be satisfied, conditions which are not always very well understood, and sometimes seem just to increase the production cost.
Cold Shortness
It is very difficult to distinguish one broken cast iron piece from another because of cold shortness, or broken due to the reduced elasticity of cast iron as compared to the other metallic materials. This may be why the fragile cold shortness of some cast iron pieces has been interpreted as a liability of cast iron, and, consequently, the finding of usability criteria for cast irons, based on cold shortness, has not been pursued.
These two ways of failure could be distinguished on the basis of the propagation rate of failure, or on the basis of the energy absorbed by the failure. By the absorption of a shock without failure, its energy passes into the piece as deformation energy, up to the limit of elasticity.
The difference between the ratio of resilience and the ratio of deformation energies appears due to the impossibility of an accurate determination of the volume of the material taking part in the absorption of energy. And this is due to properties of cast iron being not direction dependent.
However, if resiliency must remain the criterion in evaluating the brittleness of cast iron — in order to be in agreement with evaluating steel brittleness — then it will be necessary not to take the resiliency value needed for steel pieces as a criterion in evaluating the resiliency value needed for cast iron at the same working temperatures.
The temperature dependence of failure energy of different cast irons and steels is provided for comparison in Figure 1. If the failure energy of cast iron is less than that of steel at the same temperature, this does not mean that cast iron pieces cannot be used. One must reconsider the resiliency values of cast irons in the case of refrigeration. This is because, as mentioned previously, the resiliency is used in this case only for comparing different sorts of cast iron, not for comparing cast iron with steel.
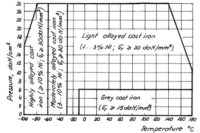
We can say that cast iron has good behavior at low temperatures, since it has no transition temperature. A not too large decrease of resiliency is observed at -80°C, but grey cast irons will not be used below -196°C. Tests showed the resiliency of pearlitic cast iron to decrease to 30 percent at -120°C as compared to 20°C, but that of cast iron with spherical graphite only to 90 percent. Whereas gray cast iron has been considered to be a brittle material, it is now generally recommended for manufacturing machinery parts working in the wall temperature interval of -15 to 250°C. Thus, gray cast irons SCe 15-32 and SCe 18-36 (GOST l4l2-54) can be used for execution of pieces from the salt solution circuit of the cooling system up to the pressure of 4 daN/cm2 and temperatures of -15°C.
The character of the structure of the parent metal and, above all, the way of graphite formation and separation determine the properties of the melting charge. The more diminished the shape of graphite, the more compact its form, and the more uniformly dissipated, the higher the resiliency. The first compound of gray cast iron — pearlite — is analogous to pearlite in steel. That ferrite in cast iron dissolves silicon in iron α. Although gray cast irons with ferrite mass resist larger shock energy and have lower transition temperatures than gray cast irons with pearlitic mass, the latter are more frequently used, since they are common pig irons.
Generally, cast irons with a small content of carbon and moderately alloyed are not resilient to cold and corrosion. They are replaced by highly alloyed cast irons in the low temperature range. The alloying elements of cast irons with increased resilience are the same as those of steel: Cr, Si, Ni, Cu, Ti, Mo. Light alloyed cast irons contain 1 percent of alloying elements, moderately alloyed ones 3 to 10 percent, and highly alloyed ones over 10 percent. By changing the composition with the help of the alloying elements, one must obtain a ferrite, pearlitic, suctionic, troostitic, martenisitic or austenitic structure of the matrix, commanding in this way the form, the quantity, and dispersion of the free graphite as desired.
Spherical graphite producing increases the resiliency at low temperatures. Thus, austenitic cast irons with spherical graphite can be used down to the temperature of -253°C. The high resilience of these cast irons at low temperatures proves the importance of separation of carbides, of the stability degree of austenite. For the diminution of transition temperature, transformation of austenite in martensite must be avoided.
Experience shows that cast iron 1 can be used to -100°C. Normalized cast iron 4, due to the high mechanical resistance and its anti-frictional properties, can be used in constructions with thin walls exposed to wear to -196°C (cylinders for compressors). Cast iron 4 can be used successfully also for pump barrels, working at temperatures of -100°C (Figure 2).
Due to its high mechanical characteristics, nodular cast iron is expected to become the only material recommended for construction of cast iron pieces for refrigeration equipment. It would be of particular benefit if one would produce crankcases for compressors, hats for heat exchangers, valve bodies, etc., since from an economic point of view, nodular cast iron has not too high a price as compared to other cast irons and steel. Waste due to hot crack, to constructional tension, etc., would be reduced substantially, providing the resilience desired at low temperatures at the thin walls. At the same time the weight of the pieces would be much smaller due to the increased resiliency as compared to cast irons with flaked graphite (Table 1).
The energy variation-temperature curves in Figure 3 have been obtained by using V-notched bars. From the shape of the curves one can observe that using low chilling temperatures for cast irons with nodular graphite, after a previous annealing, resilience and especially the transition temperature is ameliorated, as compared to other cast irons of the same chemical composition but normalized and hardened at high temperatures. Amelioration seems to result owing to the metallic mass with reduced carbon content.
Practically, in the case of cast iron one cannot speak about ductile failure, but only about a fragile one. This is because the propagation speed of failure cracks is large, but it does not produce any local deformation, only micro deformation. The tendency to fragile failure increases with decreasing temperature, with increasing deformation speed, or if special tension states (space states) are applied. Major failure of cast irons must be avoided, since such failure can appear without previous warning and usually has disastrous results.
Publication date: 9/17/2012