In this installment of the Btu Buddy series, Bob stops at a diner for lunch and has something on his mind. He has been to school where all of the terms of pressure and vacuum have been discussed, but he does not have a firm grasp on the terms. He wants to better understand the evacuation of a system. About that time Btu Buddy appears.
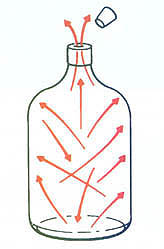
He explains, “Vapor molecules in a vessel travel in a straight line and bounce around the vessel. If there is an opening in the vessel, the molecules will begin to move out of the opening in proportion to the pressure difference from the inside of the vessel to the outside (Figure 1). There are many more molecules at a higher pressure. If the vessel is under 20 psig of pressure and atmospheric pressure is 0 psig, there is a 20 psi incentive for the vapor to move out. There are a lot of vapor molecules at 20 psig. When the vessel pressure is at 0 psig, there is not much incentive for the molecules to move out of the vessel. The vacuum pump provides the incentive by creating a low pressure area for the molecules to move to.”
Bob tells Btu Buddy, “I remember all of this in school, but I can’t say that I really understood it.”
Btu Buddy says, “To really understand what this low pressure is, you must understand the units of that pressure. It is much like going to a foreign country; you must understand the currency or you never know where you stand.”
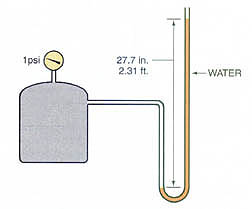
“The weatherman talks about inches of mercury (Hg) to express atmospheric pressure. We often use millimeters of mercury to express pressures in our field. The weatherman would tell you that the atmosphere will support a column of Hg that is 29.92 inches high (Figure 3). The 29.92 inches is often referred to as 30 inches. It is an equal pressure to 33.94 feet of water and is the measure of sea level atmosphere on a standard pressure day. Since these two pressures are equal, and used by everyone in our field, you must be able to convert from one to the other. In reality, 1 psi is equal to 2.036 inches of Hg. This can be verified by dividing 29.92 by 14.696, (29.92/14.696 equals 2.0359 rounded to 2.036). The 2.036 is called a conversion factor or unit.”
Btu Buddy says, “For the very reasons you just said, it is the language of HVACR.”
Bob asks, “But how are we going to use it?”
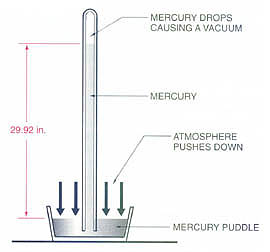
Btu Buddy says, “When you buy your instruments and vacuum pump for removing the atmosphere, all of these terms come up and more. For example, we have not even talked about the terms used to measure a deep vacuum. Remember, the atmosphere will support a column of Hg 29.92 inches high.
“In the U.S., we express positive pressures in pounds per square inch gauge. Our vacuum gauge registers pressures below atmospheric and has a vacuum scale expressed in inches of Hg vacuum. This scale is needed because it is divided into smaller increments. We talk in terms of millimeters of Hg down into the very low vacuums that must be obtained to properly evacuate a system.”
Bob says, “Yes, I know the term and cannot say that I can explain it to anyone else.”
Btu Buddy notes, “When it comes to deep vacuums, manufacturers use even more terms that you should be familiar with. Let’s say it this way: The atmosphere will also support a column of Hg 760 millimeters high. Just remember, all of the terms of measuring a column have been simple linear measurements, feet, inches, and millimeters. These terms can easily be converted from one to another. Before we put together a conversion table, we must discuss one more important term, microns. A micron is equal to 1/1,000th of a millimeter. A millimeter is about 1/2 the thickness of a dime.
“The object of reducing the pressure in a system to a deep vacuum, known as pulling a vacuum, is to remove vapor from the system. It is measured in microns. Many manufacturers will say in their specifications that they want you to pull a vacuum to 250 or 500 microns and hold that pressure for a time span to prove there are no foreign vapors in the system. This would include water vapor. A pressure reading of 500 microns is actually 1/2 of a millimeter and cannot be readily detected with the naked eye with a column of Hg.”
Btu Buddy then says, “You must realize by now that your gauge manifold will not measure pressures this low. That is why your company furnishes you with an electronic gauge to measure vacuum. It is calibrated to read in microns. Your manifold gauge could better be called an indicator. It is not a precise measuring instrument.”
Bob says, “You know, I have used that micron gauge a lot, but did not really understand what it was all about.”
Btu Buddy says, “Let’s construct a simple chart that explains the pressure conversions.”
1 inch equals 254 millimeters
1 millimeter equals 1,000 microns
1 psi equals 2.036 inches of Hg
760 millimeters equals 29.92 inches of Hg equals 14.696 psig equals 33.9 feet
Btu Buddy remarks, “When you understand the relationships of these facts, you can talk intelligently to anyone about pressures and vacuums.”
Figure 4 is a table of all of these relationships along with the saturation points for boiling water.

Btu Buddy tells Bob, “A good vacuum pump can lower the pressure to 0.1 microns. A micron is 1/1,000th of a millimeter of pressure above absolute 0 pressure expressed in inches Hg (remember, there is also pounds per square inch absolute, psia).When the vacuum pump manufacturers say this, they mean in a clean system with nothing that can boil to a vapor. This will not work in a refrigeration system because we have oil in the compressor and piping that will emit small amounts of vapor. That is why manufacturers use 250 to 500 microns.With a pressure that is this low in the vacuum pump, the system pressure is down to some very low values, assuring there is no moisture in the system after evacuation.”
Bob says, “There are sure a lot of details to evacuation. Can I ever learn them all?”
Btu Buddy says, “The learning curve is long in this business. It may never end because new technologies are introduced every day. That is what makes it an interesting career; you never see the end of the variety of knowledge. A person who is making a career of this field can learn forever. It is a constant educational challenge.”
Bill Johnson has been active in the HVACR industry since the 1950s. He graduated in gas fuel technology and refrigeration from the Southern Technical Institute, a branch of Georgia Tech (now known as Southern Polytechnic Institute). He taught HVAC classes at Coosa Valley Vocational & Technical Institute for four years. He moved on to become service manager for Layne Trane, Charlotte, N.C. He taught for 15 years at Central Piedmont Community College, part of this time as program director. He had his own business for five years doing installation and service work. Now retired, he is the author of Practical Heating Technology and Practical Cooling Technology, and continues as a co-author of Refrigeration & Air Conditioning Technology, 5th Edition, all published by Delmar Publishers. For more information, he can be reached at 704-553-0087, 704-643-3928 (fax), or bmj@myexcel.com.
Publication date: 08/25/2003