FUEL CELL SYSTEMS
The design of fuel cell systems is complex and can vary significantly depending upon fuel cell type and application. However, most fuel cell systems consist of four basic components:Most fuel cell systems also include other components and subsystems to control fuel cell humidity, temperature, gas pressure, and wastewater.
Fuel Cell Stack
The fuel cell stack is the heart of a fuel cell power system. It generates electricity in the form of direct current (DC) from chemical reactions that take place in the fuel cell. A single fuel cell produces enough electricity for only the smallest applications. Therefore, individual fuel cells are typically combined in series into a fuel cell stack. A typical fuel cell stack may consist of hundreds of fuel cells. The amount of power produced by a fuel cell depends upon several factors, such as fuel cell type, cell size, the temperature at which it operates, and the pressure at which the gases are supplied to the cell.
Fuel Processor
The fuel processor converts fuel into a form useable by the fuel cell. If hydrogen is fed to the system, a processor may not be required or it may only be needed to filter impurities out of the hydrogen gas.
If the system is powered by a hydrogen-rich conventional fuel such as methanol, gasoline, diesel, or gasified coal, a reformer is typically used to convert hydrocarbons into a gas mixture of hydrogen and carbon compounds called "reformate." In many cases, the reformate is then sent to another reactor to remove impurities, such as carbon oxides or sulfur, before it is sent to the fuel cell stack. This prevents impurities in the gas from binding with the fuel cell catalysts. This binding process is also called "poisoning" since it reduces the efficiency and life expectancy of the fuel cell.
Some fuel cells, such as molten carbonate and solid oxide fuel cells, operate at temperatures high enough that the fuel can be reformed in the fuel cell itself. This is called internal reforming. Fuel cells that use internal reforming still need traps to remove impurities from the unreformed fuel before it reaches the fuel cell.
Both internal and external reforming release carbon dioxide, but less than the amount emitted by internal combustion engines, such as those used in gasoline-powered vehicles.
Current Inverters And Conditioners
Current inverters and conditioners adapt the electrical current from the fuel cell to suit the electrical needs of the application, whether it is a simple electrical motor or a complex utility power grid.
Fuel cells produce electricity in the form of direct current (DC). In a direct current circuit, electricity flows in only one direction. The electricity in homes and workplaces is in the form of alternating current (AC), which flows in both directions on alternating cycles. If the fuel cell is used to power equipment using AC, the direct current will have to be converted to alternating current.
Both AC and DC power must be conditioned. Power conditioning includes controlling current flow (amperes), voltage, frequency, and other characteristics of the electrical current to meet the needs of the application. Conversion and conditioning reduce system efficiency only slightly, around 2 to 6 percent.
Heat Recovery System
Fuel cell systems are not primarily used to generate heat. However, since significant amounts of heat are generated by some fuel cell systems - especially those that operate at high temperatures such as solid oxide and molten carbonate systems - this excess energy can be used to produce steam or hot water or converted to electricity via a gas turbine or other technology. This increases the overall energy efficiency of the systems.
TYPES OF FUEL CELLS
Fuel cells are classified primarily by the kind of electrolyte they employ. This determines the kind of chemical reactions that take place in the cell, the kind of catalysts required, the temperature range in which the cell operates, the fuel required, and other factors. These characteristics, in turn, affect the applications for which these cells are most suitable. There are several types of fuel cells currently under development, each with its own advantages, limitations, and potential applications. They include:
Polymer electrolyte membrane (PEM) fuel cells - also called proton exchange membrane fuel cells - deliver high power density and offer the advantages of low weight and volume compared to other fuel cells. PEM fuel cells use a solid polymer as an electrolyte and porous carbon electrodes containing a platinum catalyst. They need only hydrogen, oxygen from the air, and water to operate and do not require corrosive fluids like some fuel cells. They are typically fueled with pure hydrogen supplied from storage tanks or onboard reformers.
Polymer electrolyte membrane fuel cells operate at relatively low temperatures, around 80°C (176°F). Low temperature operation allows them to start quickly (less warm-up time) and results in less wear on system components, resulting in better durability. However, it requires that a noble-metal catalyst (typically platinum) be used to separate the hydrogen's electrons and protons, adding to system cost. The platinum catalyst is also extremely sensitive to CO poisoning, making it necessary to employ an additional reactor to reduce CO in the fuel gas if the hydrogen is derived from an alcohol or hydrocarbon fuel. This also adds cost. Developers are currently exploring platinum/ruthenium catalysts that are more resistant to CO.
PEM fuel cells are used primarily for transportation applications and some stationary applications. Due to their fast startup time, low sensitivity to orientation, and favorable power-to-weight ratio, PEM fuel cells are particularly suitable for use in passenger vehicles, such as cars and buses.
A significant barrier to using these fuel cells in vehicles is hydrogen storage. Most fuel cell vehicles (FCVs) powered by pure hydrogen must store the hydrogen onboard as a compressed gas in pressurized tanks. Due to the low energy density of hydrogen, it is difficult to store enough hydrogen onboard to allow vehicles to travel the same distance as gasoline-powered vehicles before refueling, typically 300-400 miles. Higher-density liquid fuels such as methanol, ethanol, natural gas, liquefied petroleum gas, and gasoline can be used for fuel, but the vehicles must have an onboard fuel processor to reform the methanol to hydrogen. This increases costs and maintenance requirements. The reformer also releases carbon dioxide (a greenhouse gas), though less than that emitted from current gasoline-powered engines.
Direct Methanol Fuel Cells
Most fuel cells are powered by hydrogen, which can be fed to the fuel cell system directly or can be generated within the fuel cell system by reforming hydrogen-rich fuels such as methanol, ethanol, and hydrocarbon fuels. Direct methanol fuel cells (DMFCs), however, are powered by pure methanol, which is mixed with steam and fed directly to the fuel cell anode.
Direct methanol fuel cells do not have many of the fuel storage problems typical of some fuel cells since methanol has a higher energy density than hydrogen - though less than gasoline or diesel fuel. Methanol is also easier to transport and supply to the public using our current infrastructure since it is a liquid, like gasoline.
Direct methanol fuel cell technology is relatively new compared to that of fuel cells powered by pure hydrogen, and DMFC research and development is roughly 3-4 years behind that of other fuel cell types.

Alkaline fuel cells (AFCs) were one of the first fuel cell technologies developed, and they were the first type widely used in the U.S. space program to produce electrical energy and water onboard spacecraft. These fuel cells use a solution of potassium hydroxide in water as the electrolyte and can use a variety of non-precious metals as a catalyst at the anode and cathode. High-temperature AFCs operate at temperatures between 100°C and 250°C (212°F and 482°F). However, newer AFC designs operate at lower temperatures of roughly 23°C to 70°C (74°F to 158°F)
AFCs' high performance is due to the rate at which chemical reactions take place in the cell. They have also demonstrated efficiencies near 60 percent in space applications.
The disadvantage of this fuel cell type is that it is easily poisoned by carbon dioxide (CO2 ). In fact, even the small amount of CO2 in the air can affect this cell's operation, making it necessary to purify both the hydrogen and oxygen used in the cell. This purification process is costly. Susceptibility to poisoning also affects the cell's lifetime (the amount of time before it must be replaced), further adding to cost.
Cost is less of a factor for remote locations such as space or under the sea. However, to effectively compete in most mainstream commercial markets, these fuel cells will have to become more cost-effective. AFC stacks have been shown to maintain sufficiently stable operation for more than 8,000 operating hours. To be economically viable in large-scale utility applications, these fuel cells need to reach operating times exceeding 40,000 hours, something that has not yet been achieved due to material durability issues. This is possibly the most significant obstacle in commercializing this fuel cell technology.
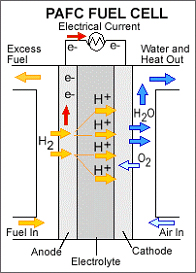
Phosphoric acid fuel cells use liquid phosphoric acid as an electrolyte - the acid is contained in a Teflon-bonded silicon carbide matrix - and porous carbon electrodes containing a platinum catalyst. The chemical reactions that take place in the cell are shown in the diagram to the right.
The phosphoric acid fuel cell (PAFC) is considered the "first generation" of modern fuel cells. It is one of the most mature cell types and the first to be used commercially, with over 200 units currently in use. This type of fuel cell is typically used for stationary power generation, but some PAFCs have been used to power large vehicles such as city buses.
PAFCs are more tolerant of impurities in fossil fuels that have been reformed into hydrogen than PEM cells, which are easily "poisoned" by carbon monoxide - carbon monoxide binds to the platinum catalyst at the anode, decreasing the fuel cell's efficiency. They are 85 percent efficient when used for the cogeneration of electricity and heat, but less efficient at generating electricity alone (37 to 42 percent). This is only slightly more efficient than combustion-based power plants, which typically operate at 33 to 35 percent efficiency. PAFCs are also less powerful than other fuel cells, given the same weight and volume. As a result, these fuel cells are typically large and heavy. PAFCs are also expensive. Like PEM fuel cells, PAFCs require an expensive platinum catalyst, which raises the cost of the fuel cell. A typical phosphoric acid fuel cell costs between $4,000 and $4,500 per kilowatt to operate.

Molten carbonate fuel cells (MCFCs) are currently being developed for natural gas and coal-based power plants for electrical utility, industrial, and military applications. MCFCs are high-temperature fuel cells that use an electrolyte composed of a molten carbonate salt mixture suspended in a porous, chemically inert ceramic lithium aluminum oxide (LiAlO2) matrix. Since they operate at extremely high temperatures of 650°C (roughly 1,200°F) and above, non-precious metals can be used as catalysts at the anode and cathode, reducing costs.
Improved efficiency is another reason MCFCs offer significant cost reductions over phosphoric acid fuel cells (PAFCs). Molten carbonate fuel cells can reach efficiencies approaching 60 percent, considerably higher than the 37-42 percent efficiencies of a phosphoric acid fuel cell plant. When the waste heat is captured and used, overall fuel efficiencies can be as high as 85 percent.
Unlike alkaline, phosphoric acid, and polymer electrolyte membrane fuel cells, MCFCs don't require an external reformer to convert more energy-dense fuels to hydrogen. Due to the high temperatures at which MCFCs operate, these fuels are converted to hydrogen within the fuel cell itself by a process called internal reforming, which also reduces cost.
Molten carbonate fuel cells are not prone to carbon monoxide or carbon dioxide "poisoning" - they can even use carbon oxides as fuel - making them more attractive for fueling with gases made from coal. Because they are more resistant to impurities than other fuel cell types, scientists believe that they could even be capable of internal reforming of coal, assuming they can be made resistant to impurities such as sulfur and particulates that result from converting coal, a dirtier fossil fuel source than many others, into hydrogen.
The primary disadvantage of current MCFC technology is durability. The high temperatures at which these cells operate and the corrosive electrolyte used accelerate component breakdown and corrosion, decreasing cell life. Scientists are currently exploring corrosion-resistant materials for components as well as fuel cell designs that increase cell life without decreasing performance.
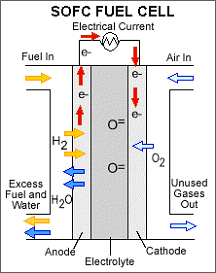
Solid oxide fuel cells (SOFCs) use a hard, non-porous ceramic compound as the electrolyte. Since the electrolyte is a solid, the cells do not have to be constructed in the plate-like configuration typical of other fuel cell types. SOFCs are expected to be around 50-60 percent efficient at converting fuel to electricity. In applications designed to capture and utilize the system's waste heat (cogeneration), overall fuel use efficiencies could top 80-85 percent.
Solid oxide fuel cells operate at very high temperatures - around 1,000°C (1,830°F). High temperature operation removes the need for precious-metal catalyst, thereby reducing cost. It also allows SOFCs to reform fuels internally, which enables the use of a variety of fuels and reduces the cost associated with adding a reformer to the system.
SOFCs are also the most sulfur-resistant fuel cell type; they can tolerate several orders of magnitude more sulfur than other cell types. In addition, they are not poisoned by carbon monoxide (CO), which can even be used as fuel. This allows SOFCs to use gases made from coal.
High-temperature operation has disadvantages. It results in a slow startup and requires significant thermal shielding to retain heat and protect personnel, which may be acceptable for utility applications but not for transportation and small portable applications. The high operating temperatures also place stringent durability requirements on materials. The development of low-cost materials with high durability at cell operating temperatures is the key technical challenge facing this technology.
Scientists are currently exploring the potential for developing lower-temperature SOFCs operating at or below 800°C that have fewer durability problems and cost less. Lower-temperature SOFCs produce less electrical power, however, and stack materials that will function in this lower temperature range have not been identified.
Regenerative Fuel Cells
Regenerative fuel cells produce electricity from hydrogen and oxygen and generate heat and water as byproducts, just like other fuel cells. However, regenerative fuel cell systems can also use electricity from solar power or some other source to divide the excess water into oxygen and hydrogen fuel - this process is called "electrolysis." This is a comparatively young fuel cell technology being developed by NASA and others.
FUEL CELL TECHNOLOGY CHALLENGES
Cost and durability are the major challenges to fuel cell commercialization. However, hurdles vary according to the application in which the technology is employed. Size, weight, and thermal and water management are also barriers to the commercialization of fuel cell technology. In stationary power applications, where cogeneration of heat and power is desired, use of PEM fuel cells would benefit from raising operating temperatures to increase performance.The key challenges include:
Excerpted from the Fuel Cells page of the U.S. Department of Energy's (DOE's) Energy Efficiency and Renewable Energy Web site. For more information, visit www.eere.energy.gov/hydrogenandfuelcells/.
Publication date: 01/09/2006






